Abstract
Introduction Iberdomide (IBER; CC-220) is an oral cereblon E3 ligase modulator (CELMoD) agent that causes degradation of Ikaros and Aiolos, transcription factors essential for lymphoma cell survival. In vitro studies suggest IBER enhances T-cell immunostimulatory and direct cytotoxic effects versus lenalidomide. IBER demonstrated apoptotic activity in diffuse large B-cell lymphoma (DLBCL) cell lines independent of cell of origin (COO). We present data from an ongoing phase 1/2 open-label study (NCT04464798) assessing safety, pharmacokinetics (PK), and preliminary efficacy of IBER alone or in combination with an anti-CD20 monoclonal antibody (mAb) in patients (pts) with relapsed or refractory (RR) lymphomas.
Methods Pts with RR lymphomas and ≥ 2 prior lines of treatment (tx) were given escalating doses of IBER alone (cohort A: all lymphomas), or with rituximab (cohort B: all B-cell lymphomas) or obinutuzumab (cohort C: marginal zone lymphoma [MZL] or grade 1-3a follicular lymphoma [FL]) to determine maximum tolerated dose (MTD). IBER starting dose was 1 mg daily (days 1-21 every 28 days) with sequential escalation to 1.3 mg and 1.6 mg. Pts received IBER for ≤ 24 cycles (12 for MZL/FL) or until disease progression or unacceptable toxicity. Pts in cohorts B and C received rituximab or obinutuzumab, respectively, for ≤ 6 cycles. Granulocyte-colony stimulating factor (G-CSF) was permitted after the dose limiting toxicity (DLT) evaluation period (tx cycle 1) was completed. Pts with measurable disease at baseline and a subsequent scan (after ≤ 3 months of tx based on clinical assessment) for response were response evaluable. The primary endpoint was MTD. Secondary endpoints included safety, PK characterization, overall response rate (ORR), and complete remission (CR) rate. Biomarker assessments were exploratory endpoints.
Results Of the 46 pts enrolled at data cutoff (March 31, 2022), 18 had RR DLBCL (cohort A, n = 8; cohort B, n = 10) and 10 had RR FL (cohort A, n = 2; cohort C, n = 8). Pts were 73% male with a median age of 69 years. Most (76%) had stage III/IV disease, while 85% had an ECOG performance status of 0-1. Median (range) prior lines of tx was 4 (1-12).
The primary reasons for tx discontinuation (49%) were progressive disease or death. Relative IBER dose intensity per cycle was maintained at 92%. No cohort achieved MTD.
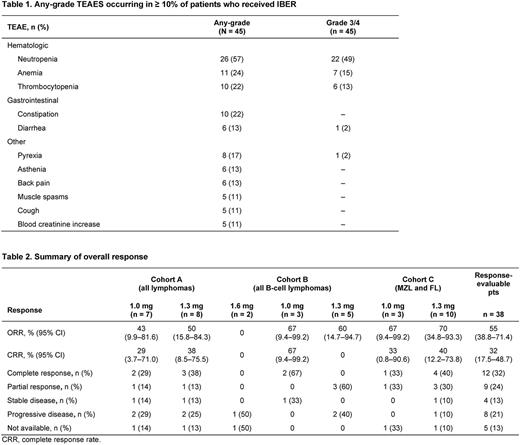
Tx-emergent adverse events (TEAEs) in ≥ 10% of pts included neutropenia, anemia, thrombocytopenia, and constipation (Table 1). Grade 3-4 TEAEs were primarily hematologic: neutropenia (49%), anemia (15%), and thrombocytopenia (13%). Febrile neutropenia occurred in 4% of pts, as did sepsis. In cohorts A and B, 1 (grade 4 thrombocytopenia) and 2 (both grade 4 neutropenia) DLTs occurred, respectively. In cohort C, 4 DLTs occurred: grade 4 neutropenia (n = 2), grade 3 facial edema (n = 1), and grade 3 hypercalcemia (n = 1). No grade 5 TEAEs occurred.
ORR in response-evaluable pts (n = 38) was 55%; 32% achieved CR (Table 2). In the 7 cohort B pts with RR DLBCL (median: 5 prior tx lines), ORR was 71%; 29% achieved CR. In cohort C (n = 13; MZL, n = 5; FL, n = 8), the ORR was 69%; CR rate was 39%. ORR and CR were independent of COO and were achieved in pts with prior exposure to lenalidomide and chimeric antigen receptor (CAR) T cell tx.
IBER was absorbed rapidly (median Tmax, 2.5-4.0 hours) and PK exposure (Cmax and area under curve) at steady state increased in a dose-related manner across the tested dose range. Consistent with preclinical data, higher doses increased Ikaros degradation in B cells (37% at 1.0 mg, 60% at 1.3 mg) but not in T cells (64% at 1.0 mg, 66% at 1.3 mg), at 6 hours post-dose. Peripheral immunophenotyping at C1D15 indicated IBER increased 2-3-fold the numbers of proliferating CD4+ T cells and activated natural killer cells. Naive T cells decreased 2-fold. Preliminary PK/pharmacodynamic analyses suggested a dose-response trend, with higher exposure related to greater reduction of Ikaros/Aiolos.
Conclusions IBER alone and in combination with anti-CD20 mAbs showed promising activity in pts with RR lymphoma. CELMoD agent-associated neutropenia was a predictable on-target toxicity manageable with G-CSF use and did not appear to increase risk of febrile neutropenia or infections. These data highlight the potential of CELMoD agent-based tx in pts with RR lymphoma to address unmet needs such as treating disease progression after CAR T cell tx.
Study support: This study was funded by Bristol Myers Squibb.
Disclosures
Thieblemont:Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support; Incyte: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Other: Travel Support; Novartis: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel Support; Bristol Myers Squibb: Consultancy, Honoraria, Other: Travel Support. Munoz:Pharmacyclics/Abbvie, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb, Kyowa, Alexion, Fosunkite, Innovent, Seattle Genetics, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, Beigene, Servier, Novartis, Morphosys/I: Consultancy; Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, Millennium: Research Funding; Targeted Oncology, OncView, Curio, Kyowa, Physicians' Education Resource, and Seattle Genetics: Honoraria; Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, Beigene, Verastem, AstraZeneca, Celgene/Bristol Myers Squibb, Genentech/Roche: Speakers Bureau. Tucci:Sanofi: Membership on an entity's Board of Directors or advisory committees; Kiowa Kyrin: Honoraria; Gentili: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria; Takeda: Honoraria. Cartron:Gilead, Novartis, Mylteni, Sanofi, Abbvie, Takeda, Roche, Janssen, Celgene, Novartis, Bristol Myers Squibb: Honoraria; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Corradini:Abbvie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GlaxoSmithKline, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Other: Data monitoring board or advisory board; Abbvie, Amgen, Bristol Myers Squibb, Celgene, Gilead/Kite, Janssen, Novartis, Roche, Takeda: Other: Support for attending meetings or travel; AbbVie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GlaxoSmithKline, Incyte, Janssen, KyowaKirin, Nerviano, Novartis, Roche, Sanofi, Takeda: Honoraria; Abbvie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GlaxoSmithKline, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Consultancy. Flinn:Novartis: Consultancy, Research Funding; Constellation Pharmaceuticals: Research Funding; Forma Therapeutics: Research Funding; Merck: Research Funding; Portola Pharmaceuticals: Research Funding; Epizyme: Research Funding; Rhizen Pharmaceuticals: Research Funding; Trillium Therapeutics: Research Funding; Century Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Genmab: Consultancy; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Iksuda Therapeutics: Consultancy; Janssen: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Nurix Therapeutics: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; CALIBR: Research Funding; Fate Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Biopath: Research Funding; Xencor: Consultancy; Roche: Consultancy, Research Funding; Millenium Pharmaceuticals: Research Funding; Infinity Pharmaceuticals: Research Funding; Incyte: Research Funding; Loxo@Lilly: Research Funding; Curis: Research Funding; Tessa Therapeutics: Research Funding; TCR2 Therapeutics: Research Funding; Secura Bio: Consultancy; Myeloid Therapeutics: Research Funding; BeiGene: Consultancy, Research Funding; City of Hope National Medical Center: Research Funding; CTI Biopharma: Research Funding; Acerta Pharma: Research Funding; Pfizer: Research Funding; IGM Biosciences: Research Funding; Forty Seven: Research Funding; Celgene: Research Funding; ArQule: Research Funding; Agios: Research Funding; InnoCare Pharma: Consultancy, Research Funding; Hutchison MediPharma: Consultancy; Gilead Sciences: Research Funding; AstraZeneca: Consultancy, Research Funding; Seattle Genetics: Research Funding; CALGB: Research Funding; Triphase Research & Development Corp: Research Funding; Unum Therapeutics: Research Funding; TG Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; Servier Pharmaceuticals: Consultancy; 2seventy bio: Research Funding. Gastinne:Gilead/Kite, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings/travel, participation in a data safety monitoring board or advisory board. Arcaini:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees. Nowakowski:Bantam Pharmaceutical: Consultancy; Blueprint Medicines Corporation: Consultancy; Celgene Corporation/Bristol Myers Squibb: Consultancy, Research Funding; Curis, Inc.: Consultancy; Daiichi Sankyo Inc: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Genentech, Inc: Consultancy, Research Funding; Incyte: Consultancy; Karyopharm: Consultancy; Kite Pharma Inc.: Consultancy; Kymera Therapeutics: Consultancy; MorphoSys US Inc: Consultancy; NanoString: Research Funding; Ryvu Therapeutics: Consultancy; Selvita: Consultancy; TG Therapeutics: Consultancy; Zai Lab: Consultancy. Topp:Gilead, Roche, Regeneron: Consultancy; Amgen, Kite, Roche, MacroGenics, Regeron: Research Funding. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. Gkasiamis:Bristol Myers Squibb: Current Employment. Drew:Bristol Myers Squibb: Current Employment. Rao:Bristol Myers Squibb: Current Employment. Kaplan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Cheng:Bristol Myers Squibb: Current Employment. Li:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Morschhauser:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal